David H. Koch (1962) Professor of Engineering
Professor of Biological and Mechanical Engineering
Associate Department Head, Biological Engineering
Member, MIT Center for Precision Cancer Medicine
Member, Ludwig Center at MIT
Member, Broad Institute of MIT and Harvard
Contact Information
Research Areas
Detection & monitoring, Metastasis, Precision medicine
Professor Manalis develops novel instrumentation for cancer research.
Research Summary
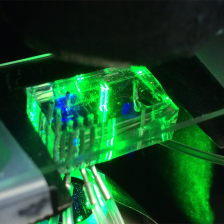
Tumors are made up of single cells. In aggregate, we know a great deal about the genetic and cellular defects that cause cancer, but comparatively little about the progress of individual cells. Our laboratory develops quantitative and real-time techniques for single cell analysis, using conventional approaches to fabricate novel fluidic devices, and exploit the unique physical properties associated with micro- and nanoscale dimensions for developing precision measurement methods.
In cancer, we are interested in creating functional assays for precision medicine. We are developing new technology platforms for predicting therapeutic response in which biophysical properties of individual tumor cells are measured in response to ex vivo treatment in a broad range of tumor types, including leukemias, glioblastoma, colon and pancreatic cancers.
Biography
Scott Manalis is the David H. Koch (1962) Professor of Engineering and faculty member in the departments of biological and mechanical engineering at MIT. He currently serves as Associate Department Head in the Department of Biological Engineering. He received a BS in physics from the University of California, Santa Barbara, and a PhD in applied physics from Stanford University. Dr. Manalis is a founder of two companies (Travera and Affinity Biosensors) that utilize the suspended microchannel resonator for weighing single cells.