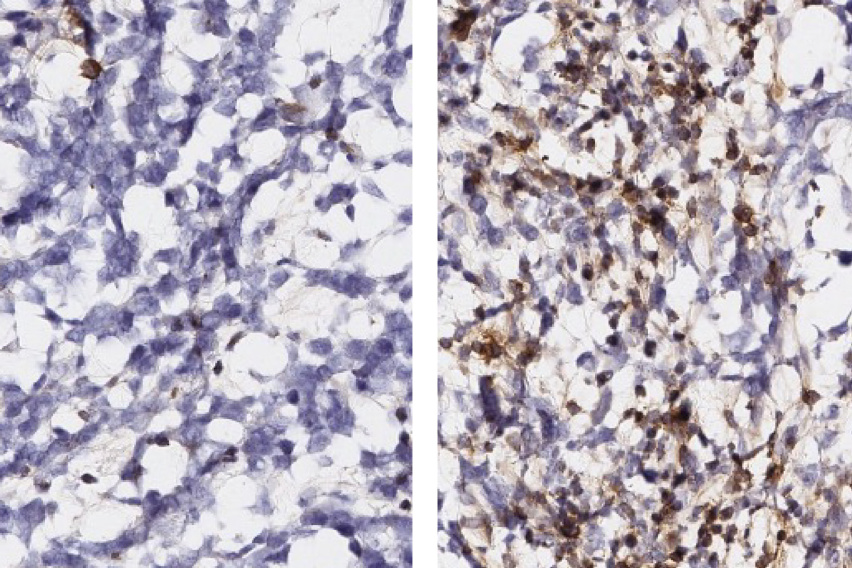
White Lab researchers and their colleagues identified peptide targets by profiling antigens in co-cultures of glioblastoma cells and macrophages, and then developed immunostimulatory treatments to test the targets’ validity. Treated mouse models of glioblastoma tumors (right) show a significantly larger infiltration of anti-tumor T cells (brown) than their untreated counterparts.
MIT Koch Institute
November 26, 2025
A study profiling antigens presented on immune and tumor cells in co-culture points to new strategies for attacking a treatment-resistant and deadly brain cancer
Glioblastoma is the most common form of brain cancer in adults, and its consequences are usually quick and fatal. After receiving standard-of-care treatment (surgery followed by radiation and chemotherapy), less than half of patients will survive longer than 15 months. Only five percent of patients survive longer than five years.
Researchers have explored immune checkpoint inhibitors as an avenue for boosting glioblastoma survival rates. This type of immunotherapy, which has proven effective against a range of tumor types, turns off a molecular switch that prevents T cells from attacking cancer cells. The patient’s own immune system is then able to clear the tumor.
However, glioblastoma is unusually resistant to attack by T cells, rendering immune checkpoint inhibitors ineffective. The culprit is a different immune cell, macrophages, which have been recruited to tumors, where they support tumor growth while suppressing the ability of T cells to infiltrate and attack tumors.
A team of researchers led by Forest White at the MIT Koch Institute for Integrative Cancer Research used sophisticated immune profiling tools to map out how macrophages evolve from a first-line defense against cancer and other pathogens into a shield that protects the glioblastoma tumor—as well as how the tumor cells themselves are transformed by the encounter.
“Looking at the co-evolution of both cell types is key,” said White, who is also the Ned C. (1949) and Janet C. (Bemis) Rice Professor in the Department of Biological Engineering. “It’s a little bit like what happens when a new family moves into a neighborhood: the family members’ lives change, but so are the social dynamics of the people around them. Whether you're mixing people or cells, you won’t be able to predict how they will interact, even if you know both well.”
“By looking at what happens when macrophages move into the tumor, we can observe changes to both types of cells that we wouldn’t otherwise be able to see,” said Yufei Cui, a PhD candidate in the White Laboratory. “We were able to identify new targets for both glioblastoma and macrophages that could be used to develop therapies that, when delivered in combination with immune checkpoint inhibitors, more effectively treat glioblastoma.”
The study, appearing in Cancer Research, includes Stefani Spranger, associate professor of biology and member of the MIT Koch Institute and Darrell Irvine, former member of the MIT Koch Institute and now professor at the Scripps Research Institute.
As in other cancers, macrophages play a pivotal role in glioblastoma development and resistance to immune therapies. In laboratory models, inhibiting the activity of tumor-associated macrophages has been found to slow glioblastoma growth, but that success has not translated to studies of human patients. While the overall strategy of targeting glioblastoma-associated macrophages is promising, new targets—derived from models that more accurately reproduce the cell interactions in patient tumors—need to be identified.
One approach to discovering such targets is a specialty of the White lab: profiling cells’ immunopeptidomes—the repertoires of antigens presented on the surfaces of cancer cells, macrophages, and many other types of cells. Surface-presenting antigens are a window into the internal state of the cell: the antigens derive from proteins produced as the cell carries out different functions and responds to its environment. By binding to surface antigens, T cells and other immune cells can monitor cells for dysfunction and respond to them.
The White lab has developed sophisticated methods for immunopeptidome profiling, combining methods such as liquid chromatography and mass spectrometry to isolate cell surface antigens—in this case from glioblastoma and macrophage cells cultured in isolation and together—and quantifying changes in expression over time. The researchers identified over 800 peptides in macrophages that either increased or decreased in expression when cultured with glioblastoma cells. Peptides with the biggest gains in expression under co-cultivation derived from 33 source proteins, mostly related to cytokine signaling that promotes tumor aggression and suppresses immune response to tumors.
Antigen presentation on glioblastoma cells was also transformed by interactions with macrophages. These antigens were associated with Rho GTPase, a signaling protein that belongs to Ras, a class of proteins that is mutated in 30% of all cancers. Changes in Rho GTPase expression predispose cells to developing hallmark traits of cancer, such as prolonged cell longevity, abnormal growth, and metastasis. Antigen profiles of co-cultured glioblastoma cells revealed over 40 Rho GTPase-associated antigens with increased expression compared to tumor cells cultured in isolation.
Researchers compared antigen expression changes in co-cultured macrophage and glioblastoma cells to immunopeptidome profiles of mouse models and human tumor samples, finding that patterns observed in cell culture translated to animal models and, potentially, to patients.
Researchers selected six antigens showing increased expression in either glioblastoma cells or macrophages to test as therapeutic targets, developing an mRNA-based immunostimulatory therapy for each antigen. After treating mice with glioblastoma, tumors showed significantly slowed growth overall and, in a few cases, were completely eradicated.
In future work, the team plans to use their immunopeptidome profiling techniques to characterize co-cultured dendritic cells, which retrieve proteins from cancer cells and presents them to T cells as antigens, as well as to explore antigen presentation of cells in live models of glioblastoma.
"This study demonstrates the promise of profiling cell surface antigens,” said Cui. "With quantitative accuracy and cell type resolution, our approach could be used to design improved immunotherapies against many cancer types and other diseases," said Cui.
This work was supported in part by the National Cancer Institute (NCI), grant #U54 CA283114, and the MIT Center for Precision Cancer Medicine.