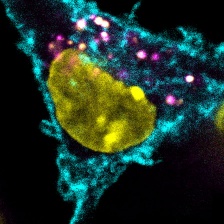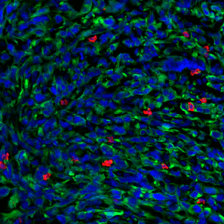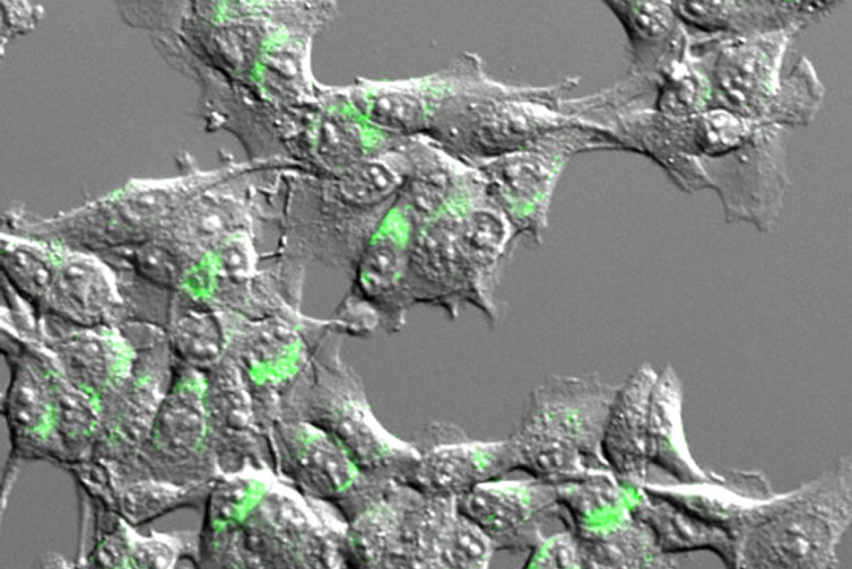
Tumor cells express a model antigen fused to a green fluorophore. Image courtesy of the researchers.
MIT Koch Institute
July 13, 2022
Immune checkpoint blockade therapies represent one of the most promising developments in cancer research and treatment, enabling cytotoxic killer T cells to attack and destroy tumor cells by removing their molecular “brakes.” While this class of immunotherapy can produce dramatic results in some cases, it remains ineffective in some patients and tumor types for reasons that are still poorly understood.
A team of researchers led by Koch Institute faculty member Stefani Spranger, the Howard S. (1953) and Linda B. Stern Career Development Professor, in collaboration with Forest White, the Ned C. (1949) and Janet C. (Bemis) Rice Professor, is shedding more light on the underlying biological mechanisms that lead to successful responses to checkpoint blockade therapy. Supported in part by the Koch Institute’s Frontier Research Program, via the Kathy and Curt Marble Cancer Research Fund, their work centers on the role of cross-presenting dendritic cells and the types of antigens they present to killer T cells.
Before T cells can mount an anti-tumor response, they have to be “trained" by dendritic cells. Dendritic cells break down detritus from dead and dying cells into protein fragments, displaying the resulting peptides on antigens to T cells. If a dendritic cell presents an antigen unique to cancer cells, then interacting T cells will be able to recognize and attack cancer cells that also bear that antigen.
While there are many different tumor-derived antigens, T cells are activated to respond only to a small fraction of them. To help account for this disparity, the Spranger and White Labs developed a method that, for the first time, surveys the number and types of antigens cross-presented by dendritic cells. In a study published in the Journal for ImmunoTherapy of Cancer, the team adapted mass spectrometry techniques developed in the White Lab for creating accurate profiles of protein fragments presented on cell surfaces. However, because dendritic cells present antigens derived from their own proteins and from dead cell debris alike, the researchers needed a way to distinguish between the two sources. To do this, they used stable isotope labeling techniques in cultured cells, flagging proteins derived from cancer cells before they are taken up by dendritic cells.
Using the new method, the researchers found that the original location of the protein within the cell made a profound difference to whether the resulting antigen would be available for cross-presentation to T cells. Antigens originating from the cytosol (the liquid filling the body of the cell) were far more likely to be cross-presented by dendritic cells than those that were once embedded in cell membrane.
The study’s results suggest that the method could help researchers understand why some patients and tumor types do not respond to checkpoint therapies, and develop new therapeutic strategies for eliciting a strong immune response. In an analysis of tissue samples from melanoma patients, the team found that the origin of putative tumor antigens could impact treatment outcomes; patients whose tumors had high ratio of membrane-derived antigens to cytosol-derived antigens tended to respond less well to checkpoint blockade therapy. In order to elicit a stronger immune response in such cases, the researchers propose that mRNA cancer vaccines encoding for antigens derived from plasma membrane proteins could be used to counteract the bias observed during the activation of T cells, making checkpoint blockade therapy a more viable treatment option for a larger group of cancer patients.